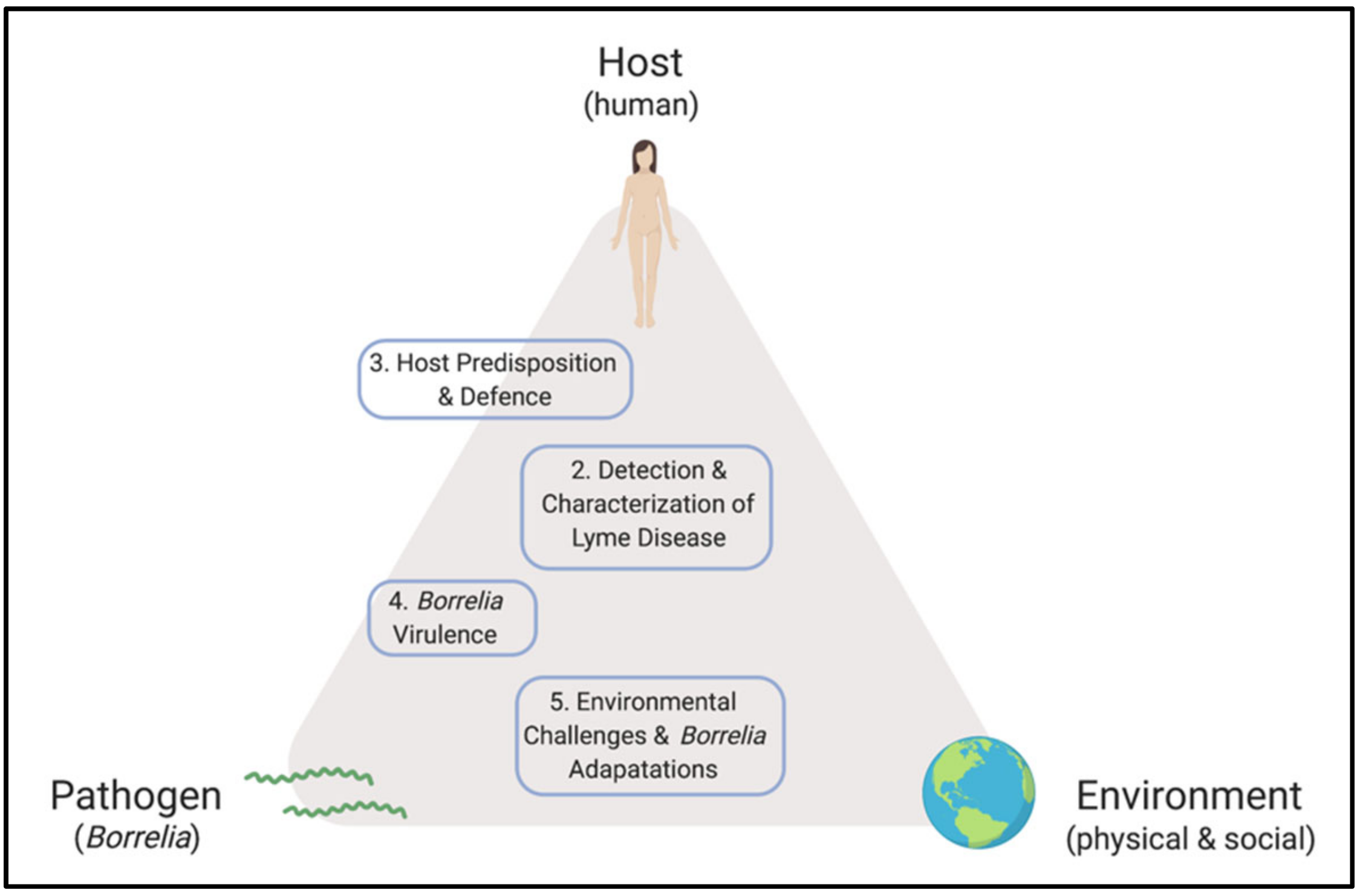
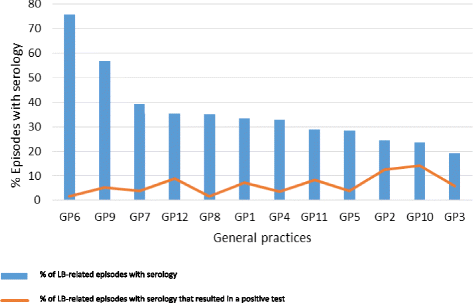
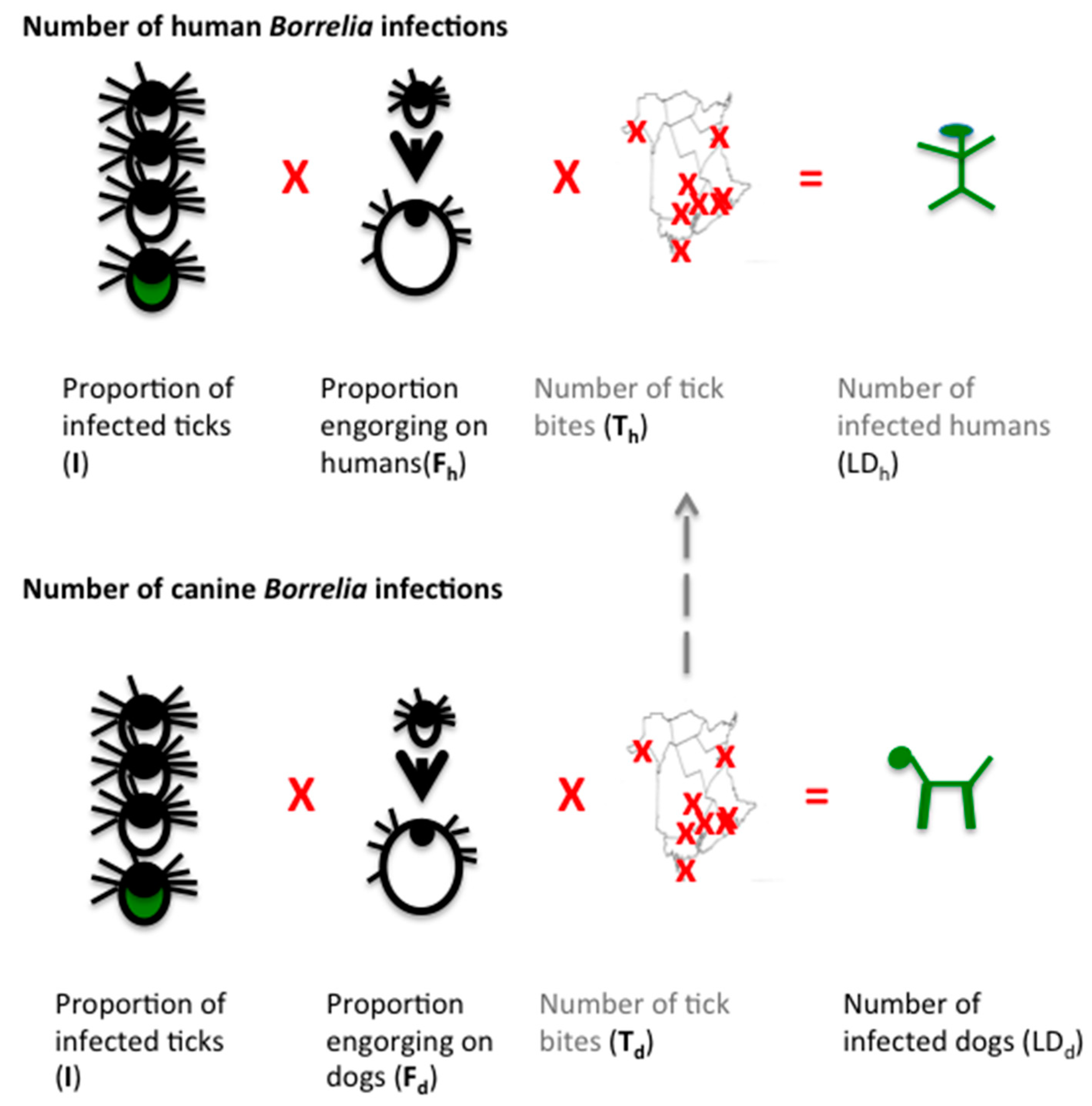
A New Screening Test For Lyme Disease Is Developed For Use In The General Population
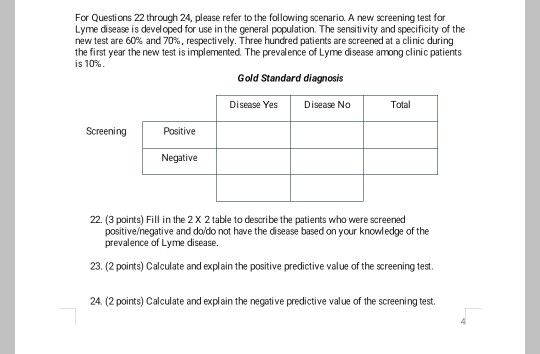
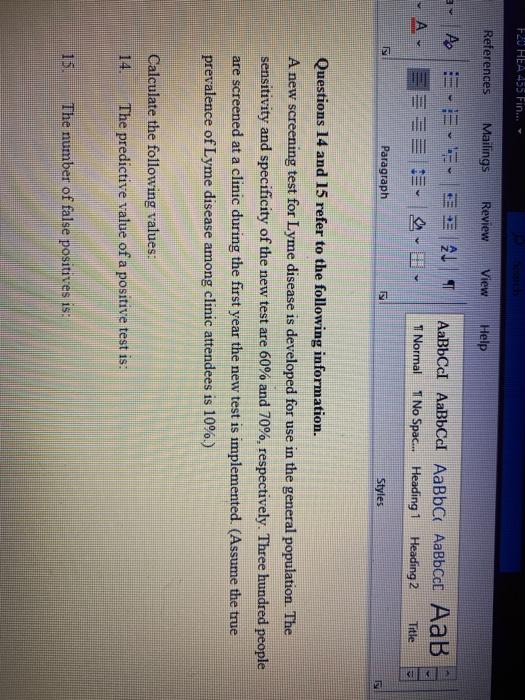
A new screening test for lyme disease is developed for use in the general population. Three hundred people are screened at a clinic during the first year the new test is implemented. A new screening test for Lyme disease is developed for use in the general population. Researchers plan to conduct a clinical trial.
Assume the true prevalence of Lyme disease among clinic attendees is 10. A new screening test for Lyme disease is developed for use in the general population. The sensitivity and specificity of the new test are 60 and 70 respectively.
Presently theres only one Food and Drug Administration-approved laboratory test for Lyme disease. A blood test that relies on detecting antibodies proteins the bodys immune system makes in. Rajadas and Pothineni have patented the compound for the treatment of Lyme disease and are working with a company to develop an oral form of the drug.
The sensitivity and specificity of the new test are 60 and 70 respectively. The sensitivity and specificity of the new test are 60 and 70 respectively. The sensitivity and specificity of the new test are 60 and 70 respectively.
Scientists are developing a new way to test early for Lyme disease using a signature of molecules in patients blood. As Pothineni said in the Stanford Medicine news release. Assume the true prevalence of Lyme disease among clinic attendees is 10.
What is a Lyme disease antibody test. An enzyme immunoassay EIA screening test. A new screening test for Lyme disease is developed for use in the general population.
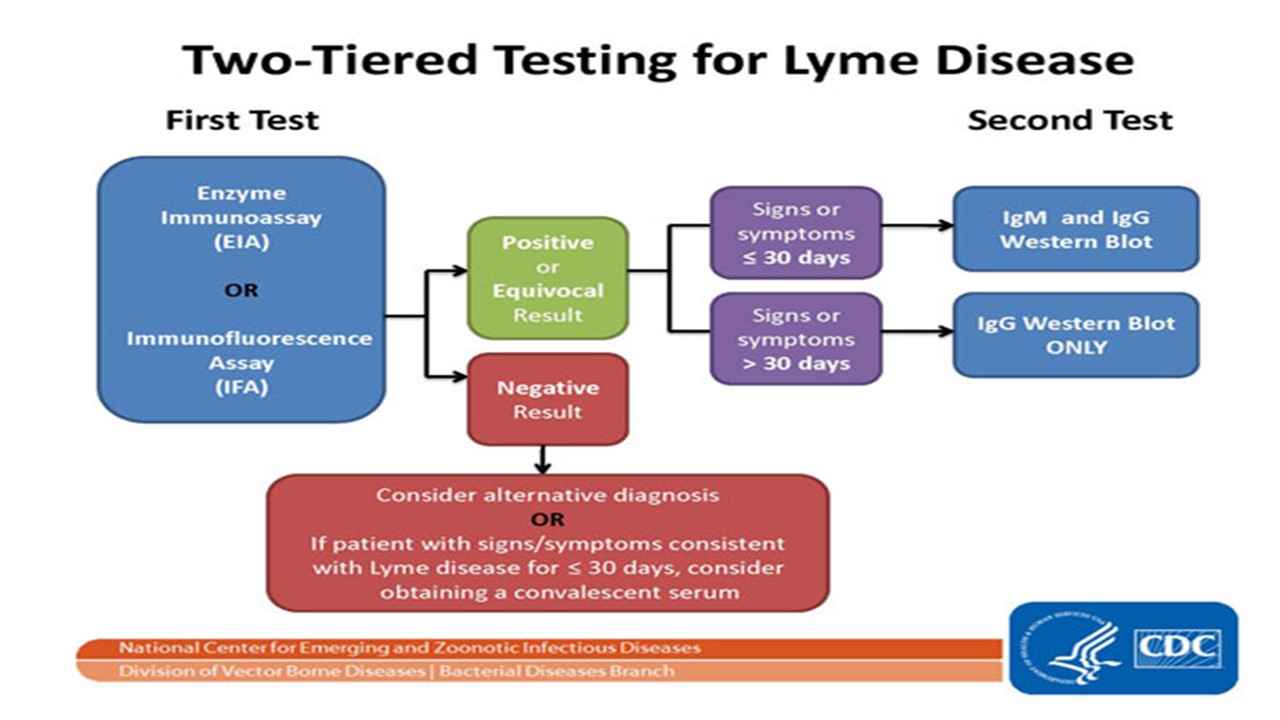
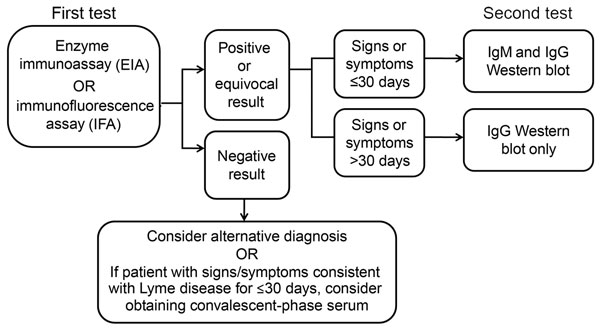
Laboratory diagnosis of Lyme disease has traditionally used a two-tier process for detecting the presence of antibodies against Borrelia burgdorferi in a patients blood. Serological testing for Lyme disease in the UK and much of the world follows a two-step approach using commonly available antibody screening tests as a.
A new screening test for Lyme disease is developed for use in the general population.
Assume the true prevalence of Lyme disease among clinic attendees is 10. A new screening test for Lyme disease is developed for use in the general population. The sensitivity and specificity of the new test are 60 and 70 respectively. The sensitivity and specificity of the new test are 60 and 70 respectively. Three hundred people are screened at a clinic during the first year the new test is implemented. The sensitivity and specificity of the new test are 60 and 70 respectively. Following the first trial will be another year-long trial that will test how long the antibody remains in the bloodstream to remain effective against future Lyme exposure. Scientists are developing a new way to test early for Lyme disease using a signature of molecules in patients blood. Laboratory diagnosis of Lyme disease has traditionally used a two-tier process for detecting the presence of antibodies against Borrelia burgdorferi in a patients blood.
Researchers plan to conduct a clinical trial. What is a Lyme disease antibody test. Assume the true prevalence of Lyme disease among clinic attendees is 10. Serological testing for Lyme disease in the UK and much of the world follows a two-step approach using commonly available antibody screening tests as a. A team led by Sam Sia professor of biomedical engineering at Columbia Engineering has developed a rapid microfluidic test that can detect Lyme disease with similar performance as. The sensitivity and specificity of the new test are 60 and 70 respectively. The sensitivity and specificity of the new test are 60 and 70 respectively.



































Post a Comment for "A New Screening Test For Lyme Disease Is Developed For Use In The General Population"